“Immune thrombocytopenia (ITP) Pipeline Insight, 2020” report by DelveInsight outlays comprehensive insights of present clinical development scenario and growth prospects across the Immune thrombocytopenia (ITP) market.
A detailed picture of the Immune thrombocytopenia (ITP) pipeline landscape is provided, which includes the disease overview and Immune thrombocytopenia (ITP) treatment guidelines. The assessment part of the report embraces in-depth Immune thrombocytopenia (ITP) commercial assessment and clinical assessment of the Immune thrombocytopenia (ITP) pipeline products from the pre-clinical developmental phase to the marketed phase. In the report, a detailed description of the drug is proffered including mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, Immune thrombocytopenia (ITP) collaborations, licensing, mergers and acquisition, funding, designations, and other product-related details.
Immune Thrombocytopenia (ITP) Treatment
The US Food and Drug Administration (FDA) has approved three Thrombopoietin receptor agonist (TPO-RA) therapies: romiplostim (Nplate), eltrombopag (Promacta), and avatrombopag (Doptelet). For Europe and Japan, only two TPO-RAs is approved, i.e., Nplate and Promacta. Promacta and Nplate will lose their patent in 2022 in the US, whereas, in Europe and Japan, Nplate loses the patent in 2019 and Promacta will lose patent in 2025. Due to their patent expiry, it is expected that the approval of Doptelet is likely to cover a major patient pool.
The major goal for the treatment of ITP is to provide a platelet count that prevents major bleeding rather than correcting the platelet count to normal levels. The management of ITP varies widely and current international guidelines recommend several first- and second-line options, including some medicinal products that have not been approved in the EU for this particular condition.
Click to know more about report.
Key Findings
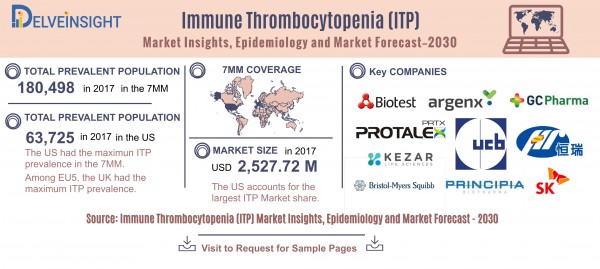
The market size of ITP in the 7MM was found to be USD 2,527.72 million in 2017.
The United States accounts for the highest market size of Immune Thrombocytopenia (ITP), in comparison to the other major markets i.e., EU5 countries, and Japan
Among the EU5 countries, the UK had the highest market size with USD 247.89 million in 2017, while Spain had the lowest market size of ITP with USD 115.08 million in 2017.
From 2023, the market value will start declining due to the patent expiration and subsequent launch of generic/biosimilar of Nplate and Promacta.
So, till 2022 Doptelet will continue to compete with both these drugs and after the patent expiry, Doptelet will not have any competitor in the market. Doptelet has contributed USD 15.50 million of the total market size of the disease in 2019.

Immune Thrombocytopenia (ITP) Marketed Drugs
Doptelet (Avatrombopag): Dova Pharmaceuticals
Tavalisse (fostamatinib disodium hexahydrate): Rigel Pharmaceuticals
Nplate (AMG-531): Amgen
Immune Thrombocytopenia (ITP) Emerging Drugs
BT-595: Biotest
Rozanolixizumab: UCB Biopharma
Efgartigimod (ARGX-113): Argenx
Key Companies in the Immune Thrombocytopenia Market
Key pharma companies fuelling the Immune thrombocytopenia (ITP) market are Octapharma USA, Amgen, CSL Behring, Dova Pharmaceuticals, Rigel Pharmaceuticals, Zenyaku Kogyo/Biogen Idec, Novartis, Bio Products Laboratory’s, Takeda Pharmaceutical Company Limited, AstraZeneca, Biotest, GC Pharma, SK Plasma, Jiangsu HengRui Medicine, Argenx, Genosco (a US-based subsidiary of Korean bio company Oscotec), Kezar Life Sciences, UCB Biopharma, Bristol-Myers Squibb, Principia Biopharma, Protalex and others.
Table of contents
1. Report Introduction
2. Immune thrombocytopenia (ITP)
3. Immune thrombocytopenia (ITP) Current Treatment Patterns
4. Immune thrombocytopenia (ITP) – DelveInsight’s Analytical Perspective
5. Therapeutic Assessment
6. Immune thrombocytopenia (ITP) Late Stage Products (Phase-III)
7. Immune thrombocytopenia (ITP) Mid Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Immune thrombocytopenia (ITP) Discontinued Products
13. Immune thrombocytopenia (ITP) Product Profiles
14. Immune thrombocytopenia (ITP) Key Companies
15. Immune thrombocytopenia (ITP) Key Products
16. Dormant and Discontinued Products
17. Immune thrombocytopenia (ITP) Unmet Needs
18. Immune thrombocytopenia (ITP) Future Perspectives
19. Immune thrombocytopenia (ITP) Analyst Review
20. Appendix
21. Report Methodology
About DelveInsight
DelveInsight is a premier Business Consulting and Market Research firm focused exclusively on the life science segment. With a wide array of smart end-to-end solutions, the firm helps the global Pharmaceutical and Bio-Tech companies formulate prudent business decisions for better growth in the market.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Vinita Rakheja
Email: Send Email
Phone: 9193216187
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: www.delveinsight.com/

