
DelveInsight’s Marburg Virus Disease pipeline report covers the Marburg Virus Disease pipeline landscape in full, with in-depth data on 6+ companies and 6+ pipeline drugs. The pipeline drug profiles contain both clinical and nonclinical stage drugs. It also looks at drugs by product type, stage, administration route, and molecular type.
Some of the Important Findings from the Marburg Virus Disease Pipeline Report
-
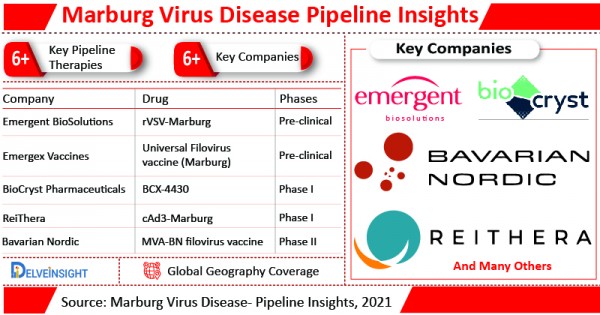
There are currently 6+ Marburg Virus Disease pipeline therapies in various stages of development.
-
MVA-BN filovirus vaccine is the pipeline therapy for Marburg Virus Disease, which is currently in Phase II of clinical trials.
-
BioCryst is developing Galidesivir (BCX-4430) in collaboration with U.S. government agencies and other institutions, which is currently in Phase I of clinical trials. Other pipeline therapy which is in Phase I of clinical trials include cAd3-Marburg.
-
Some pipeline therapies of Marburg Virus Disease such as Universal Filovirus vaccine (Marburg) and rVSV-Marburg are still in the early stage of development i.e. pre-clinical stage.
-
Bavarian Nordic, BioCryst Pharmaceuticals, ReiThera, Emergex Vaccines, Emergent BioSolutions and others are among the leading companies which are developing potential drug candidates to boost the Marburg Virus Disease treatment scenario.
For further information request sample @ Marburg Virus Disease Pipeline Analysis
Marburg Virus Disease: Overview
Marburg Virus Disease (Marburg Hemorrhagic Fever) is a severe illness that affects humans and nonhuman primates and is caused by one of two marburgviruses, Marburg virus (MARV) or Ravn virus (RAVV). Marburg Virus Disease is a viral hemorrhagic fever (VHF) with clinical symptoms similar to Ebola Virus Disease (EVD).
Marburg Virus Disease: Symptoms
After a 5-10 day incubation period, symptom onset is abrupt and is characterised by fever, chills, headache, and myalgia. A maculopapular rash, most prominent on the trunk (chest, back, stomach), may appear around the fifth day after the onset of symptoms. Nausea, vomiting, chest pain, sore throat, abdominal pain, and diarrhoea may occur. Jaundice, pancreatic inflammation, severe weight loss, delirium, shock, liver failure, massive haemorrhaging, and multi-organ dysfunction are all possible symptoms.
Marburg Virus Disease: Treatment
Marburg Hemorrhagic Fever has no specific treatment. Supportive hospital therapy, such as balancing the patient’s fluids and electrolytes, maintaining oxygen status and blood pressure, replacing lost blood and clotting factors, and treating any complicating infections, should be used.
Marburg Virus Disease Pipeline Analysis: Drug Profiles
BCX-4430: BioCryst Pharmaceuticals
Product Description
Galidesivir (BCX4430) is a broad-spectrum antiviral in advanced development for the treatment of viruses that endanger public health and national security, such as SARS-CoV-2 (the cause of COVID-19), Ebola, Marburg, Yellow Fever, and Zika.
Phase I
In December, 2018, BioCryst Pharmaceuticals began “A Phase 1 Double-blind, Placebo Controlled, Dose Ranging Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Galidesivir (BCX4430) Administered as Single Doses Via Intravenous Infusion in Healthy Subjects”. This is a placebo-controlled, randomised, double-blind study to assess the pharmacokinetics of galidesivir after a single IV infusion. The trial was completed in April 2019 with 32 enrolled participants.
Results
-
Galidesivir was found to be safe and well tolerated in healthy subjects in Phase 1 clinical safety and pharmacokinetics trials using both intravenous and intramuscular routes of administration. Galidesivir has shown survival benefits in animal studies against a variety of serious pathogens, including Ebola, Marburg, Yellow Fever, and Zika viruses.
-
Galidesivir has also shown broad-spectrum activity in vitro against over 20 RNA viruses from nine different families, including coronaviruses, filoviruses, togaviruses, bunyaviruses, arenaviruses, paramyxoviruses, and flaviviruses.
Get in touch to know more about the Marburg Virus Disease Pipeline therapies.
Marburg Virus Disease Pipeline Therapies and Key Companies
-
MVA-BN filovirus vaccine: Bavarian Nordic
-
cAd3-Marburg: ReiThera
-
BCX-4430: BioCryst Pharmaceuticals
-
Universal Filovirus vaccine (Marburg): Emergex Vaccines
-
rVSV-Marburg: Emergent BioSolutions
Find out more @ Marburg Virus Disease Pipeline Therapies and Key Players
Marburg Virus Disease Therapeutics Assessment
-
By Product Type
-
Monotherapy
-
Combination Therapy
-
By Stage
-
Discovery
-
Pre-Clinical
-
Phase I
-
Phase II
-
Phase III
-
Pre-registration
-
By Route of Administration
-
Inhalation
-
Intravenous
-
Oral
-
Subcutaneous
-
By Molecule Type
-
Small Molecule
-
Stem Cell Therapy
-
Gene Therapy
-
Targets
-
Immune System
-
Multiple Kinase
-
By Mechanism of Action
-
Protease Inhibitors
-
Immunomodulatory
Scope of the Report
Coverage: Global
Marburg Virus Disease Key Players: Bavarian Nordic, BioCryst Pharmaceuticals, ReiThera, Emergex Vaccines, Emergent BioSolutions, among others
Marburg Virus Disease Pipeline Therapies: MVA-BN filovirus vaccine, cAd3-Marburg, BCX-4430, Universal Filovirus vaccine (Marburg), rVSV-Marburg, and others
Table of Contents
|
1. |
Introduction |
|
2. |
Executive Summary |
|
3. |
Marburg Virus Disease: Overview |
|
4. |
Marburg Virus Disease- Analytical Perspective In-depth Commercial Assessment |
|
5. |
Marburg Virus Disease Pipeline Therapeutics |
|
6. |
Marburg Virus Disease Late Stage Products (Phase III) |
|
7. |
Marburg Virus Disease Mid Stage Products (Phase II) |
|
8. |
Marburg Virus DiseaseEarly Stage Products (Phase I) |
|
9. |
Marburg Virus Disease Preclinical Stage Products |
|
10. |
Marburg Virus Disease Therapeutic Assessment |
|
11. |
Marburg Virus Disease Inactive Products |
|
12. |
Marburg Virus DiseaseCompany-University Collaborations (Licensing/Partnering) Analysis |
|
13. |
Marburg Virus Disease Key Companies |
|
14. |
Marburg Virus Disease Key Products |
|
15. |
Marburg Virus Disease- Unmet Needs |
|
16. |
Marburg Virus Disease- Market Drivers and Barriers |
|
17. |
Marburg Virus Disease- Future Perspectives and Conclusion |
|
18. |
Marburg Virus Disease Analyst Views |
|
19. |
Appendix |
|
20. |
About DelveInsight |
For rich insights into Healthcare and Pharmaceutical News, visit Pharma, Healthcare and Biotech Blog Posts
Key questions answered in the Marburg Virus Disease Pipeline Report
-
What are the different Marburg Virus Disease treatment options?
-
How many pharmaceutical companies are working on treatments for the Marburg Virus?
-
Which of these companies’ therapies is the most widely used?
-
What is the total number of drugs manufactured by each company for the Marburg Virus Disease?
-
How many Marburg Virus Disease pipeline therapies are in the early, mid, and late stages of development?
-
How many of the in-development therapies can be used alone or in combination with other treatments?
-
What are the most important industry-industry and industry-academy cooperation, mergers and acquisitions, and licensure practises that have an impact on Marburg Virus Disease?
Related Reports
Get comprehensive analysis of Ebola Fever pipeline therapies and key companies including Jansen Pharmaceuticals, CanSino Biologics, Galactica Biotech Ltd, Gilead Sciences, GlaxoSmithKline, Johnson & Johnson, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing end to end comprehensive solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Sandeep Joshi
Email: Send Email
Phone: 9193216187
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: www.delveinsight.com/

